November 16, 2022, The Meals and Drug Administration (FDA) accepted lab-grown meat merchandise by UPSIDE Meals after rigorous testing to make sure they’re protected for human consumption. It’s a huge step within the lab grown meat business and it’s the joint effort of FDA, United States Division of Agriculture’s Meals Security and Inspection Service (USDA-FSIS) and the producers to guarantee the security and legality of lab-grown meat following the Federal Meals, Drug, and Beauty Act (FD&C) [1].
The FDA is accountable for facility inspections and pre-market session within the regulating course of. Throughout the inspection, a consultant of the FDA is anticipated to be on website to observe the entire manufacturing course of from starting to finish. What’s extra, they plan to carry out one other inspection when the corporate launches the industrial product to have a greater understanding on potential security hazards [1]. Pre-market session is a course of that assesses the meals security of lab grown meat earlier than it’s put available on the market. The builders collaborate with the FDA and advise them of the considerations they need to concentrate on to make make sure the manufacturing complies with the usual of the FD&C’s necessities [1].
There are 4 predominant checkpoints recognized through the manufacturing course of: cell assortment, cell line and financial institution, differentiation, and harvest. The FDA has jurisdiction and regulates the primary three processes, whereas the USDA-FSIS takes over regulation of harvesting and labeling [1]. Throughout the cell assortment course of, sampled cells are collected both from grownup chickens destined for human consumption or mid-stage fertilized hen eggs. Some potential dangers embody sourcing the wrong animal and pathogen contamination from both the surface setting or an animal’s compromised well being situation. Due to this fact, the FDA will evaluate the strategy of cell isolation and identification and check to make sure pathogens are current [1].
Cell line and financial institution is when the sampled cells are screened, grown to a bigger amount, and saved for later use. UPSIDE screens the cells based mostly on the next standards:
- The capability to develop repeatedly whereas sustaining a steady phenotype.
- The capability to distinguish into muscle (myoblasts) or connective tissue (fibroblasts) cells.
- The capability to develop in suspension medium with out the necessity for floor attachment (As a result of the expansion would happen in a tank filled with cultured media).
- The flexibility to develop on strong substrates afterwards and type an intact muscle product.
Aside from potential pathogen contamination, some unintended genetic change may happen as a result of multiplication. Due to this fact, UPSIDE would use know-how like PCR to verify the soundness of the genetics, and the FDA would study the corporate’s high quality management process together with figuring out the cell, monitoring pathogen contamination and assessing cell growth [1].
A certain quantity of cells are pulled from the cell financial institution and put right into a bioreactor to a number of into muscle tissue with vitamins equipped through the differentiation stage. Noting that the cells are positioned in a small vessel with cultured media at the start, after few rounds of cell division, the cells shall be transferred to a bigger vessel. This course of is named passaging and is both carried out underneath sterile circumstances or single-use meals grade vessels may be employed, with the classy media continuously underneath agitation to verify the cells are suspended homogeneously [1].
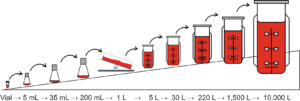
Determine 1: Passaging course of [2]
The method is very prone to pathogen contamination throughout quite a few instances of passaging or brought on by doubtlessly insufficient sterilization of the bioreactor. What’s extra, a few of the media elements is likely to be current as residues through the harvesting course of. To handle the chance, UPSIDE implements a monitoring program. Since cultured media is essential for the wholesome progress of the cell, the FDA evaluates the substance within the tradition media together with vitamins, progress elements (the factor that alerts the expansion and differentiation of cells), and buffer, amongst different issues. The FDA additionally evaluates the potential hazards in every manufacturing step, the agency’s contingency plan and the standard management measurement [1].
Lastly, when the cells are grown to the anticipated density, they’re harvested from the sealed vessel. The regulatory oversight handed from FDA to USDA in analyzing the composition, potential residue and contaminants could be assessed. The services additionally must get a USDA inspection grant and cling to USDA-FSIS rules, for instance, setting sanitation requirements and placing into place HACCP (Hazard Evaluation and Important Management Factors) techniques [1].
The purpose is to control human meals constructed from cultivating animal cells based mostly on the prevailing regulation set for historically produced meat. The Federal Meat Inspection Act (FMIA), if the animal is cattle, or the Poultry Merchandise Inspection Act (PPIA), whether it is poultry, governs the slaughter and processing of livestock within the originating jurisdiction. On this case, the farmed meat should additionally adhere to FMIA and PPIA rules [1].
What’s subsequent
After the regulatory social gathering approves the sale, the lab-grown meat business continues to be surrounded by unanswered questions. For instance, customers’ willingness to do this product is unclear, contemplating customers’ previous resistance to different meals technological advances like genetically modified meals. Affordability may be one other concern whereas the business continues to be exploring methods to scale up manufacturing [4].
In response to UPSIDE Meals, the hen would first be obtainable in Michelin’s three-star restaurant, Atelier Crenn, earlier than transferring on to grocery shops [4]. Equally, when Singapore change into the primary nation that authorizes the sale of lab-grown meat, US-based lab-grown meat startup Eat Simply additionally offered its lab-grown hen to high-end eating places and inns [3].

Determine 2: Instagram person Ted Cowan posted photographs of the dishes and the menu, which confirmed {that a} trio of cultured meat dishes price S$23 [3].
Concentrating on premium eating places is an excellent alternative as a result of the novel and pricy nature of lab-grown meat matches the tone that premium eating places attempt to set up. Moreover, meat dishes are a staple of many various cultures’ cuisines. Due to this fact, the evolution of meat dishes displays the progress of society and civilization.
We all the time entangle ourselves in an ethical dilemma, the place slaughter appears to be the one and solely solution to acquire meat. However as lab-grown meat turns into actuality, our urge for food for meat and animal welfare can lastly coexist. It is a illustration of how technological developments can act to free humanity from moral conflicts.
Reference:
[1] Heart for Meals Security and Utilized Vitamin. (2022, November 16). Human meals made with cultured animal cells. U.S. Meals and Drug Administration. Retrieved December 23, 2022, from https://www.fda.gov/meals/food-ingredients-packaging/human-food-made-cultured-animal-cells
[2] Hernández Rodríguez, T., & Frahm, B. (2020). Design, Optimization, and Adaptive Management of Cell Tradition Seed Trains. Strategies in Molecular Biology, 2095, 251–267. https://doi.org/10.1007/978-1-0716-0191-4_14
[3] Thiagarajan, S. (2020, December 21). Lab-grown hen meat now obtainable at Eatery in Robertson Quay. Mothership.SG – Information from Singapore, Asia and world wide. Retrieved December 23, 2022, from https://mothership.sg/2020/12/eat-just-good-meat-launch-singapore/
[4] Toeniskoetter, C. (2022, November 17). Lab-grown meat receives clearance from F.D.A. The New York Occasions. Retrieved December 23, 2022, from https://www.nytimes.com/2022/11/17/local weather/fda-lab-grown-cultivated-meat.html
 Concerning the Writer:
Concerning the Writer:
Xanxin is at the moment pursuing her undergraduate diploma in Meals Science on the College of Guelph. Wanxin additionally goes by the title Maggie, as a result of her dad and mom met one another at Maggi seasoning sauce plant and fell in love, which set the tone for Wanxin selecting Meals Science as her research and profession alternative. Wanxin can be the President of the Meals Science membership on the College of Guelph, having fun with constructing the neighborhood of foodies and serving to folks know extra about Meals Science. Rising up in China and coming to Canada to check, Wanxin develops a robust appreciation in direction of totally different cultures. She loves touring, making new mates and exploring native tradition by way of the delicacies. In her free time, it’s also possible to discover her juggling.

